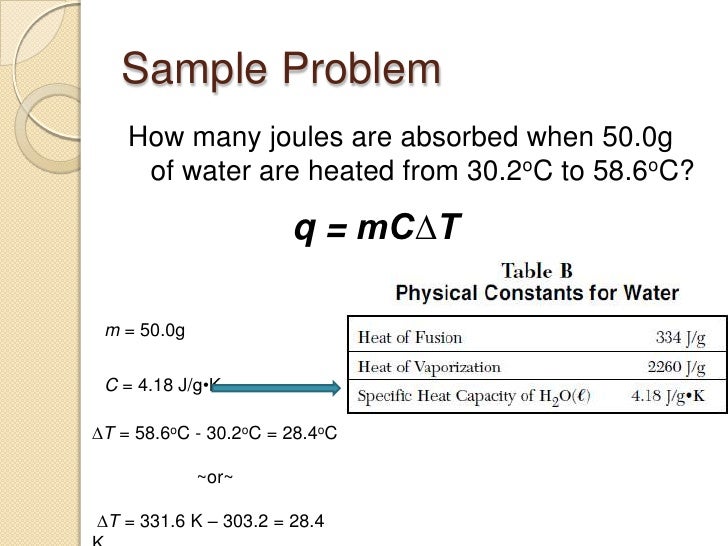
The lab was done by using a plastic bag, with baking soda and vinegar reacting to produce carbon dioxide gas to inflate the bag. In order to calculate the correct grams of baking soda as well as the correct milliliters of vinegar we had to use math (stoich). The first step I used was to fill the container with water and use that amount to figure out the amount of volume available in the plastic bag. After this was done, I used pressure x volume in liters divided by .0812 x 294.15 kelvin = moles of carbon dioxide gas, I then used the mole number of carbon dioxide to work backwards to get the grams of baking soda. I then used the grams of baking soda which is the limiting reagent, and figured out the ml of vinegar that needed to be used. Overall the lab was a success and I passed the lab! lol
Airbag Video
Online lab help

So in our last unit of the school year we are learning about gas laws. The first gas law that we talked about in this unit is Boyle's law. In this gas law the temperature is kept at a constant. Therefore the pressure of a gas varys as well as the pressure. The rule of thumb is that as the pressure of the gas goes up the volume of the container goes down. The reason why this happens is the gas particles have less room to move therefore hitting the side of the container more often which increases the pressure. The standard formula for Boyle's law is P1(v1)=P2(v2).


http://www.one-school.net/Malaysia/UniversityandCollege/SPM/revisioncard/physics/heat/gaslaw.html
https://www.youtube.com/watch?v=N5xft2fIqQU