The half life of a substance is the time it takes for the sample to decay into a stable form. This is shown in the following graph.
The following video is very helpful to better understand how to calculate half life: https://www.youtube.com/watch?v=DfXYy10Vqlo
Tuesday, September 29, 2015
Radioactivity
We learned about radioactivity and the three types of decay: alpha, beta, and gamma. The following chart is a summary from my notes.
A video to help explain the calculations behind tracking decay series is https://www.khanacademy.org/science/chemistry/nuclear-chemistry/radioactive-decay/v/alpha-beta-and-gamma-decay
Tuesday, September 22, 2015
Beanium Lab
We completed a lab in class today to deepen our knowledge and practice our skills of solving average atomic mass. The scenario we were given had us conducting follow up experiments on a newly discovered element, "Beanium", which uniquely has atoms that are very large and isotopes can be sorted by hand. My lab partner and I counted total number of atoms in the sample, then sorted the atoms by isotope, counting the number of atoms for each isotope, and determined mass using a scale. Using this information, we calculated average mass for each isotope by dividing the number of atoms for each isotope by the total mass of each isotope. After, we calculated the percent abundance by dividing the number of atoms for each isotope by the number of atoms on the whole sample. We then filled in the average mass and percent abundance of each isotope into the equation for average atomic mass. This is found in an earlier post of mine, Isotopes, it also provides a little more background information about the whole process of finding atomic mass.
 |
| Materials for lab |
New Project
We were assigned a new project to create an online database tracking the elements in the stars. Astronomy has never been something I was very interested in, but I hope I can learn some things and gain a little more interest and appreciation for it all at the end of this project. Some links I uswede to help me find the information are: http://www.astronoo.com/en/brightest-stars.html, http://www.astronoo.com/en/stars.html, and http://www.umop.net/spctelem.htm. These links help me first to identify and star and it's stellar classification, then to identify the chemical make-up, and last to find the visual spectrum of the most abundant element.
Isotopes
Monday in class, we learned about isotopes. Isotopes are two or more forms of the same element containing the same number of protons, but different number of neutrons. Neutrons are responsible for giving an element it's mass; therefore, isotopes have different atomic masses. To calculate average atomic mass of all isotopes for a particular element, the mass of each isotope is multiplied by it's percent abundance and then added together. For a deeper explanation and more clarification on the topic, I found the following video very helpful. Atomic Mass: Introduction
Monday, September 21, 2015
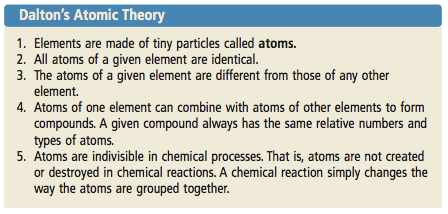
Atomic Theory
We started our new unit today about Atomic Structure and Radioactivity. We began exploring some theories about the structure of the atoms and the experiments done to prove these theories.
Thomson discovered the electron by using a cathode ray tube to show atoms of an element emit particles with a negative charge. He made the chocolate chip cookie model
Rutherford discovered the proton through his gold foil experiment and proved the presence of a positively charged center in an atom. A visual representation of this is:
Tuesday, September 15, 2015
Pre-Test
Today I took a pre-test for the new unit about Atomic Structure and Radioactivity, I noticed I struggled a lot on all the questions and I knew nothing about what was being asked. However, this makes me excited about all the new things I can and get to learn about in the upcoming unit. I feel like this unit will be more difficult than the previous unit about nomenclature because I had a little bit of background knowledge before going into that, that I feel I don't have for this unit.
Monday, September 14, 2015
Naming Acids
I recently learned about naming acids. I found a very helpful flowchart to easily follow along and name them.
Naming Compounds
I learned how to name types 1, 2, and 3 binary compounds. I found this very useful flowchart that with a few easy steps can help me name compounds.
Some important notes to remember are when naming Type 1 are, the cation (metal) is named first and uses it's element name. The anion ( non-metal) is named second and the ending of the element name is removed and replaced with the suffix -ide. With type 2 naming, it is important to remember that the roman numeral following the transition metal represents the charge and not the number of atoms. Type 3 naming the first element is named using full element name, but the second element is named as an anion. The chart below can help you with prefixes.
Be sure to make sure that the words agree such that if there is oo, drop the first o and same with ao, drop the a.
Be sure to make sure that the words agree such that if there is oo, drop the first o and same with ao, drop the a.
Subscribe to:
Comments (Atom)