We learned periodic trends, and putting them to practice with a few activities. The first trend is atomic size, where atoms get larger because you move down a group, and also get bigger as you move from right to left, leaving the largest atoms in the bottom left corner. The next trend is ionization energy, which is the energy needed to remove an electron from a gaseous state atom, and it results in forming a cation. This trend is at its greatest in the top right. The third trend regarding only the s and p blocks, along with the two previous trends is electron affinity, which is the ease with which an electron may be added to an atom, creating an anion, otherwise, the energy given off when an electron joins an atom, and this gives off negative energy. This trend is also at its greatest in the top right. The final trend we learned about in our supplement is electronegativity, which is the tendency of an atom to draw electrons toward itself when chemically combined with another element, and this is greatest to the right and up, but does not include noble gases.
This overall demonstrates all the trends:
Here are some practice links:
http://www.acschools.org/cms/lib07/PA01916405/Centricity/Domain/362/Periodic%20Trends%20Worksheet%20Answers.pdf
http://www.sfponline.org/uploads/71/periodictrendspracticesub1106.pdf




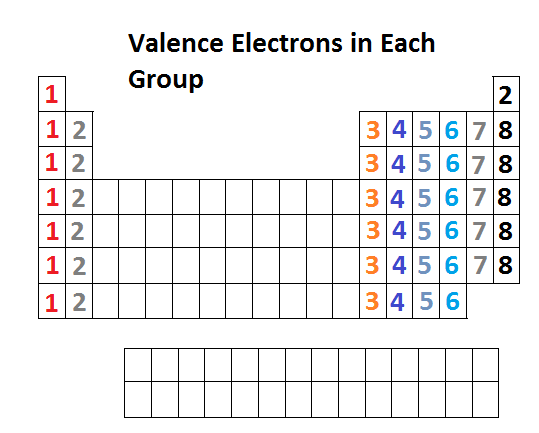 Octet Rule
Octet Rule